Research and development item: ③(b) Development of mathematical models of toxicokinetics and biological responses of nanomaterials
Implemented by the National Institute of Advanced Industrial Science and Technology
Final objective: We describe the results of inhalation toxicity testing and intratracheal administration testing conducted within the scope of the project using mathematical models, and establish them as general physiological mathematical models that describe the relationship between toxicokinetics and biological responses of the nanomaterials.
Main results:
(1) Pulmonary clearance of nanomaterials and translocation to other organs after intratracheal administration
To understand the hazard posed by exposure to nanomaterials through the respiratory route, information related to the toxicokinetics of nanomaterials, i.e., retention in the lung and translocation to other organs, is important. Therefore, we administered nanomaterials intratracheally to rats; collected samples of the lung, BALF (bronchoalveolar lavage fluid), lung-related lymph nodes, and other main organs temporally; measured the nanomaterials in each organ using analytical methods such as ICP-MS (inductively coupled plasma mass spectrometry); and analyzed the results using mathematical models.
To clarify the difference in the toxicokinetics of various nanomaterials, we evaluated the results of intratracheal administration testing of several types of TiO2 nanomaterials, NiO nanomaterials, and SiO2 nanomaterials (research and development item ①).
Figure ③(b)-1 shows a comparison of the pulmonary clearance rate after intratracheal administration of seven types of TiO2 nanomaterials with different physicochemical properties to rats. Differences in size and shape did not significantly affect pulmonary clearance, except in the case of the material with Al(OH)3 surface coating (“TTO-S-3 with coating” in the figure), which exhibited a low clearance rate. Irrespective of the material, the pulmonary clearance rate tended to decrease at larger doses (Shinohara et al. in print).
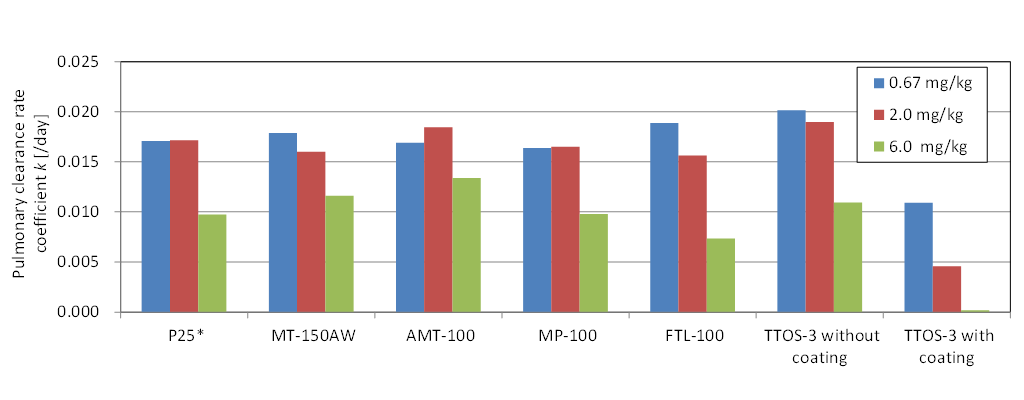 Figure③(b)-1 Pulmonary clearance rate of TiO2 nanomaterials with different physicochemical properties (* in the case of P25, the dose was 0.75, 1.5, and 6.0 mg/kg-body weight)
Figure③(b)-1 Pulmonary clearance rate of TiO2 nanomaterials with different physicochemical properties (* in the case of P25, the dose was 0.75, 1.5, and 6.0 mg/kg-body weight)
(2) Distribution to main organs in intravenous administration testing
In general, only small amounts of nanomaterials that reach the lung through the respiratory route translocate from the lung to other organs; quantitative analysis of the quantity of migrated material is difficult.
To study the toxicokinetics of nanomaterials after they enter the body and the blood flow, we conducted intravenous administration testing to evaluate their distribution to the main organs and the concentration decay in the organs. Thus far, we tested several types of TiO2 and NiO nanomaterials.
Figure ③(b)-2 shows results of testing in which single administration of TiO2 nanomaterials was performed to the tail vein of a rat at a dose of 1 mg/kg-body weight. Observations were made up to one month after the administration. The organs to which the nanomaterials were distributed after administration were primarily the liver and the spleen. The concentration decay in these organs was slow (Shinohara et al. 2014b).
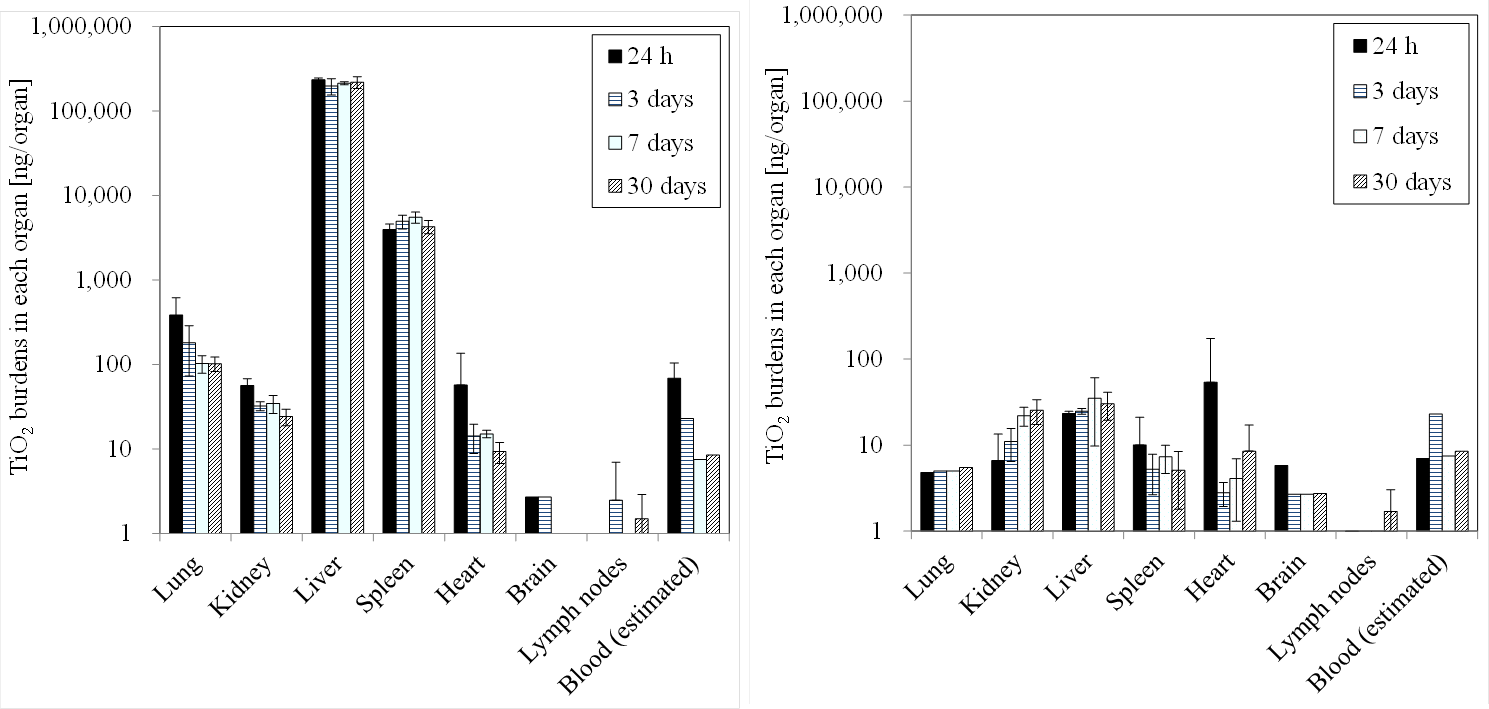 Figure③(b)-2 Results of intravenous administration testing of TiO2 nanomaterials: distribution to main organs and concentration decay
Figure③(b)-2 Results of intravenous administration testing of TiO2 nanomaterials: distribution to main organs and concentration decay
(3) Local pulmonary distribution of nanomaterials after intratracheal administration
Research and development item ② compares results of inhalation toxicity testing and intratracheal administration testing. One issue concerning intratracheal administration testing is that the local distribution of nanomaterials in the lung may be different from that in the case of inhalation exposure.
To quantitatively examine the local distribution of nanomaterials in the lung of an exposed rat, we observed lung tissue sections using a fluorescence X-ray microscope (Zhang et al. 2015 ).
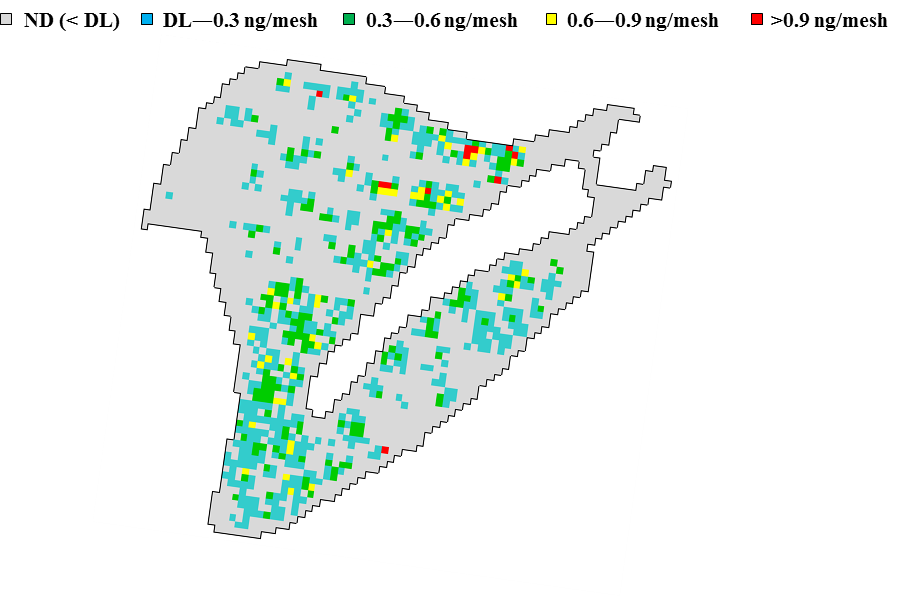 Figure③(b)-3 Titanium distribution in the lung (right caudal lobe) of a rat to which TiO2 nanomaterials were administered intratracheally (dose of 3.3 mg/kg × three times (total 10 mg/kg))
Figure③(b)-3 Titanium distribution in the lung (right caudal lobe) of a rat to which TiO2 nanomaterials were administered intratracheally (dose of 3.3 mg/kg × three times (total 10 mg/kg))
Figure ③(b)-3 shows an observation image, with 100-µm mesh resolution, of a tissue section of right caudal lobe of the lung of a rat to which TiO2 nanomaterials were administered intratracheally at 10 mg/kg-body weight. It shows areas of high titanium concentration and areas of titanium concentration similar to those of the background.
We used this technique to compare intratracheal administration with inhalation exposure as well as to compare single intratracheal administration with multiple intratracheal administrations. Moreover, we used an immunostained tissue section to study the relationship between the localization of nanomaterials and the accumulation of macrophages in the lung.
